°î(d®Γng)«ΑΈΜ÷Ο > Ήμ™(y®®) > ΦΦ–g(sh®¥)ΈΡ’¬ > MSDκäΜ·¨W(xu®Π)Αl(f®Γ)ΙβΦΦ–g(sh®¥)Ε®ΝΩ…ζΈοΙΛΥ΅÷–ΒΡκsΌ|(zh®§)
MSDκäΜ·¨W(xu®Π)Αl(f®Γ)ΙβΦΦ–g(sh®¥)Ε®ΝΩ…ζΈοΙΛΥ΅÷–ΒΡκsΌ|(zh®§)
ΓΨ…ζΈοΥéèS…ζ°a(ch®Θn)Ό|(zh®§)ΩΊ≤ΩιTΘ§…ζΈοΥéCMOΤσ‰I(y®®)ΓΩΚ±“ä≤ΓΥéèS Ι”ΟECLΦΦ–g(sh®¥)Ε®ΝΩ…ζΈοΙΛΥ΅ΒΡκsΌ|(zh®§)
Implementation of electrochemiluminescence technology for quantification of bioprocess impurities
Ι”ΟκäΜ·¨W(xu®Π)Αl(f®Γ)ΙβΦΦ–g(sh®¥)ECLΕ®ΝΩ…ζΈοΙΛΥ΅÷–ΒΡκsΌ|(zh®§)
Αl(f®Γ)±μïr(sh®Σ)ιgΘΚ2008Ρξ
Ής’ΏÜΈΈΜΘΚûθΤ’Υ_ά≠¥σ¨W(xu®Π)Θ®»πΒδ’Z(y®≥)ûιUppsala universitetΘ©Θ§ΈΜΨ”»πΒδΒΎ“ΜΘ§öW÷όΒΎΕΰ °“ΜΦΑ άΫγΒΎΤΏ °“ΜΓΘ
¨ç(sh®Σ)ρû(y®Λn)ÜΈΈΜΘΚSwedish Orphan BiovitrumΙΪΥΨΘ®SobiΘ©Θ§§F(xi®Λn) –÷Β43É|Οά‘ΣΓΘ2015ΡξΘ§ίx»π“βàD ’Όè(g®Αu)Ώ@Φ“»πΒδ―–Αl(f®Γ)÷Έ·üΚ±“ä≤ΓΥéΈοΒΡ÷ΤΥéΙΪΥΨΓΘΤδ÷ς“Σ―–÷ΤΚ±“ä≤ΓΥéΈοΘ§Αϋά®ΑΉ―Σ≤ΓΓΔΉ‘…μΟβ“Ώ–‘Φ≤≤ΓΓΔ¥ζ÷x–‘Φ≤≤ΓΓΔΑ©ΑY÷ß≥÷·üΖ®Β»ΓΘ÷ς“ΣΖ÷≤Φ‘ΎöW÷όΓΔ±±ΟάΓΔΑΡ¥σάϊ¹ÜΓΔ–¬ΈςΧmΓΘΙΪΥΨ‘Ύ¨ΘΩΤΥéνI(l®Ϊng)”ρΏM(j®§n)––ι_Αl(f®Γ)Θ§Αϋά®―ΉΑY/Οβ“ΏΦ≤≤ΓΓΔ»ήΟΗσwΌAΖeΑYΓΔ―Σ“Κ≤ΓΓΔΖ ≈÷ΑYΓΔΒΑΑΉΧφ¥ζ·üΖ®Β»ΓΘ
2013 Ρξ 1 ‘¬ 8 »’Θ§Swedish Orphan BiovitrumΘ®SobiΘ©ΙΪΥΨ–ϊ≤ΦΘ§Οά΅χ(gu®°) ≥ΤΖΥéΤΖ±O(ji®Γn)ΕΫΙήάμΨ÷Θ®FDAΘ©“―≈ζ€ (zh®≥n)KineretΘ®anakinraΘ§ΑΔΡ«ΑΉ€ΰΥΊΘ©”Ο”ΎÉΚΆ·≈c≥…»ΥΒΡ‘γΑl(f®Γ)–‘ΕύœΒΫy(t®·ng)―ΉΑY–‘Φ≤≤ΓΘ®NOMIDΘ©÷Έ·üΓΘ±ΨΤΖ≥…ûιΝΥΒΎ“Μ“≤ «Έ®“Μ“ΜΖNΫ¦(j®©ng) FDA ≈ζ€ (zh®≥n)ΒΡ NOMID ÷Έ·üΥéΈοΘ§NOMID «cryopyrinΒΑΑΉœύξP(gu®Γn)ΒΡ÷ήΤΎ–‘ΨCΚœ’ςΘ®CAPSΘ©÷–Ήνûι΅ά(y®Δn)÷ΊΒΡΑY†νΓΘΏ@ «±ΨΤΖΒΎ“Μ¥ΈΪ@≈ζ”Ο”ΎÉΚΆ·ΜΦ’Ώ÷–Θ§÷°«Α“≤“―Ϊ@ΒΟΝΥ FDA ΒΡΙ¬ÉΚΥé’J(r®®n)Ε®ΓΘΡΩ«ΑቨΠ(du®§) NOMID ΜΦ’Ώ¦]”–Ώm“ΥΒΡ·üΖ®Θ§ΕχΜυ”Ύ‘™ΥéΈο‘Ύ·ü–ßΖΫΟφΒΡΨό¥σù™ΝΠΘ§±ΨΤΖ±Μ Ύ”ηΝΥÉû(y®≠u)œ»¨è≤ιôύ(qu®Δn)ΓΘ‘γ‘Ύ 2001 ΡξΘ§±ΨΤΖΨΆΪ@≈ζ”Ο”Ύ≥…»ΥνêοL(f®Ξng)ùώ–‘ξP(gu®Γn)Ιù(ji®Π)―ΉΘ®RAΘ©ΒΡ÷Έ·üΓΘ
CAPS «“ΜΖNΑικSΫK…μ≤Δïΰ(hu®§)“ΐΤπ»Υσw΅ά(y®Δn)÷ΊΥΞ»θΒΡΦ≤≤ΓΘ§NOMID ³t «‘™νê³e÷–Ήνûι΅ά(y®Δn)÷ΊΒΡΑY†νΘ§≈cΑΉΫιΥΊ -1Θ®IL-1Θ©Οβ“ΏœΒΫy(t®·ng)ΒΑΑΉΒΡΏ^Ε»Ζ÷ΟΎ”–ξP(gu®Γn)ΓΘκS÷χ≤Γ«ιΒΡΏM(j®§n)’ΙΘ§ΜΦ’ΏΒΡ¬†ΝΠΚΆ“ïΝΠ÷πùu€pΆΥΘ§’J(r®®n)÷ΣΙΠΡή°a(ch®Θn)…ζ’œΒKΘ§≤Δ“ΐΤπ≤ΜΆ§≥ΧΕ»ΒΡξP(gu®Γn)Ιù(ji®Π)ΉÉ–ΈΓΘ‘™ΥéΈοιL(zh®Θng)Ώ_(d®Δ) 5 ΡξΒΡ―–ΨΩ±μΟςΘ§±ΨΤΖ≥ΐΝΥΡήâρΩΊ÷Τ»’≥ΘΑY†νΘ§»γΑl(f®Γ)üαΓΔΤΛ’νΓΔν^Ά¥ΓΔξP(gu®Γn)Ιù(ji®Π)Ά¥Β»Θ§¨Π(du®§)”ΎΖÄ(w®ßn)Ε®÷–‰–…ώΫ¦(j®©ng)œΒΫy(t®·ng)Θ®CNSΘ©ΒΡΙΠΡήΘ®»γ¬†ΝΠΚΆ“ïΝΠΒ»Θ©”–÷χ¥_«–ΒΡ·ü–ßΓΘ
κäΜ·¨W(xu®Π)Αl(f®Γ)ΙβΦΦ–g(sh®¥)ΘΚ¹μ(l®Δi)Ή‘Οά΅χ(gu®°)Meso Scale DiscoveryΓΘ
―–ΨΩΡΩΒΡΘΚ Ι”ΟMSD ECLΧφ¥ζELISAΘ§ ΙΒΟôz€y(c®®)ΗϋΩλΘ§ΗϋΚΟΓΘ
In recombinant protein manufacturing for the purpose of producing protein-based drugs it is important to be able to demonstrate that impurities have been efficiently removed during the purification process and that the final product is pure. This project is involved in quantification of some of the impurities that may be found in a biopharmaceutical product; insulin, insulin like growth factor 1 (IGF-1) and host cell proteins (HCP). Analysis techniques that are to be used have to meet high demands in both sensitivity and in precision. Today the process related impurities are mainly analyzed with enzyme linked immunosorbent assays (ELISAs) that are available commercially as kits. However a new technique developed by Meso Scale Discovery (MSD) where the detection is based on electrochemiluminescence (ECL) technology is starting to replace ELISA. The reasons why the ECL assay may be superior to ELISA is that the measuring range is wider and the detection limit of the technique often is much lower, which means that the sensitivity is increased. Also the possibility to test multiple analytes in the same assay, a multiplex assay, reduces time and work load. This project evaluates this new technology by comparing results from the ECL assay with ELISA. Initially with one analyte at the time and if this comparison is successful the assays will be tested in a multiplex ECL assay format.
ΫY(ji®Π)’™:
• The ECL assay is suitable for insulin and more sensitive than the ELISA currently used.
MSDκäΜ·¨W(xu®Π)Αl(f®Γ)ΙβΦΦ–g(sh®¥)±»ELISAΗϋΏm”Ο”Ύ“»çuΥΊôz€y(c®®)Θ§Τδλ`ΟτΕ»±»Τδ Ι”ΟΒΡELISAΗΏ
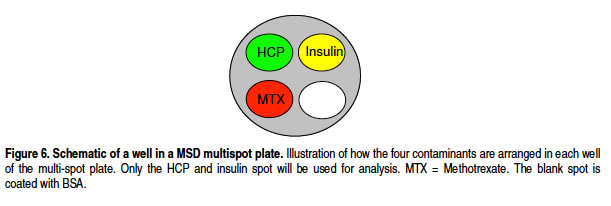
• The singleplex and duplex assay for HCP generate similar signals.
Υό÷ςΦö(x®§)ΑϊΒΑΑΉHCPΒΡÜΈ“ρΉ”ôz€y(c®®)ΚΆΕύ“ρΉ”ôz€y(c®®)–≈Χ•(h®Λo)“Μ÷¬( Ι”ΟMSDΕύ“ρΉ”ΦΦ–g(sh®¥)Ω…“‘‘Ύ“Μ²Ä(g®®)ΩΉÉ»(n®®i)Ά§ïr(sh®Σ)ôz€y(c®®)Υό÷ςΦö(x®§)ΑϊΒΑΑΉHCPΚΆΥéΈοΘ§Ιù(ji®Π)Φsïr(sh®Σ)ιgΚΆ³ΎΝΠΘ©
’™ΈΡ»ΪΈΡΩ…Ά®Ώ^ΜΊèΆ(f®¥)ύ]ΦΰΥς“ΣΘ§ΓΘ
- –g(sh®¥)÷–≥…œώ–¬ΤΣ’¬ΘΚΙΧëB(t®Λi)ΑΉΙβΙβ‘¥“ΐνI(l®Ϊng)αt(y®©)·ü’’ΟςΗο–¬
- ΙΧëB(t®Λi)’’ΟςΦΦ–g(sh®¥)Ηο–¬Εύ¬ΖèΆ(f®¥)”Οü…Ιβôz€y(c®®)
- ΙΧëB(t®Λi)Ιβ‘¥ϋc(di®Θn)ΝΝü…Ιβ‘≠ΈΜκsΫΜΦΦ–g(sh®¥)--Χα…ΐ…ζΈοαt(y®©)¨W(xu®Π)―–ΨΩΚΆ≈R¥≤‘\îύ–¬Ώx™ώ
- σw≤Φά≠ΗώΙβ•≈Θ®VBGsΘ©‘ΎΝΩΉ”Ιβ¨W(xu®Π)÷–ΒΡëΣ(y®©ng)”Ο
- ÷–κAΧίΙβ•≈ΙβΉVÉxΦΑΤδëΣ(y®©ng)”Ο
- ΧΫΟΊ¥≈–‘≤ΡΝœΒΡ…ώΤφ÷°ΙβΓΣΓΣ¥≈ΙβΩΥ†•
- Pt/C¥ΏΜ·³©–¬ΦΦ–g(sh®¥)ΫιΫBΘΚ Ι”ΟΝςΜ·¥≤ ALD ÷Τ²δΗΏ–‘Ρή»ΦΝœκä≥Ί¥ΏΜ·³©
- κäâΚιTΩΊ–‘β}Ά®ΒάΠΝ2ΠΡ¹ÜΜυ-1’{(di®Λo)ΩΊΫY(ji®Π)ΡcΑ©ΏM(j®§n)’Ι≤Δ”ΑμëΤδΈΔ≠h(hu®Δn)Ψ≥
- IEA/Kingfar Award»Υ“ρ≈cΙΛ–ß¨W(xu®Π)―–ΨΩΣ³(ji®Θng)≈e––νCΣ³(ji®Θng)Éx Ϋ
- üαΝ“ëcΉΘüo(w®≤)εaΡΆΥΦΙP ΫΉΔ…δΤς Ή¥Έ≥ωΩΎΟά΅χ(gu®°)
- ξΜΝΩïr(sh®Σ)ιgœύξP(gu®Γn)ÜΈΙβΉ””΄(j®§)îΒ(sh®¥)ΤςTime Tagger v2.17Αl(f®Γ)≤Φ
- …œΚΘ–¬÷ZÉxΤς÷ζΝΠÖf(xi®Π)όkΒΎ °“Μ¨Ο…œΚΘ ––¬≤ΡΝœ³™(chu®Λng)–¬¥σΌê
- ΉVηDΙβκäΆΤ≥ωΫY(ji®Π)‰΄(g®Αu)Ψo€ê–ΆοwΟκΟ}¦_€y(c®®)ΝΩ?j®©)xFROG
- ξΜΝΩΙβκä¥ζάμ…ΧΓΣΖ®΅χ(gu®°)ALPAO–¬ΤΖΉÉ–ΈγRDM57-15…œΨÄ
- Ν_ί΄―ϊΡζÖΔΦ”÷–΅χ(gu®°)…ώΫ¦(j®©ng)ΩΤ¨W(xu®Π)¨W(xu®Π)ïΰ(hu®§)ΒΎ °ΤΏ¨Ο»Ϊ΅χ(gu®°)¨W(xu®Π)–g(sh®¥)ïΰ(hu®§)Ήh
- ûIΥ…―ϊΡζÖΔ’Ι2024Ρξ…νέΎΒΎ25¨Ο÷–΅χ(gu®°)΅χ(gu®°)κHΙβ≤©ïΰ(hu®§)
Copyright(C) 1998-2024 …ζΈοΤς≤ΡΨW(w®Θng) κä‘£ΘΚ021-64166852;13621656896 E-mailΘΚinfo@bio-equip.com